KB004 (Anti-EphA3) Program
Humaneered®, Recombinant Monoclonal Antibody for the Treatment of Cancers
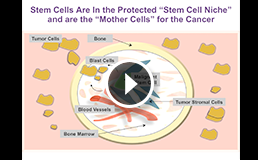
KB004 Targets Hematologic Malignancies, Solid Tumors, and their Stem Cells
KB004 is a first-in-class, monoclonal antibody targeting the EphA3 receptor tyrosine kinase created using KaloBios’ proprietary Humaneered® technology. KB004 is unique in its potential to attack tumors at their source by killing tumor cells, tumor stromal cells that protect them, and the vasculature that feeds them without killing normal cells. This unique combination of activities may provide the potential to generate durable responses. KB004 may have a similar mechanism of action in patients with solid tumors since it targets solid tumor stem cells and their microenvironment.
Clinical Development Status: Phase 2 of Phase 1/2 Clinical Trial in Hematologic Malignancies Currently Enrolling Patients with Myelofibrosis or Myelodysplastic Syndrome
KaloBios is currently conducting a Phase 1/2 study evaluating KB004 in hematologic malignancies in patients who are prescreened prior to treatment for EphA3 positivity. A biomarker assay has been developed to identify EphA3 expression on bone marrow sample from patients.
KaloBios completed the Phase 1 dose-escalating portion of this clinical trial in hematologic malignancies at multiple sites in the U.S. and Australia. Data from the Phase 1 portion of this clinical trial shows that KB004 is well tolerated and has promising clinical activity. Despite the fact that this Phase 1 clinical trial was an "all-comers" clinical trial where patients were not being screened for EphA3 expression, and the fact that more than 80% of patients enrolled were late-stage AML patients, there were three responders; one AML patient who experienced a complete response with incomplete platelet recovery (CRp), and two clinical improvements, as defined by international guidelines, one in a patient with myelodysplastic syndrome and one in a myelofibrosis patient. An improvement in collagen fibrosis was also associated with these responses. The most common safety event associated with KB004 administration in the current patient population being tested has been infusion reactions. These data were presented in a poster presentation at the American Society of Hematology (ASH) Annual Conference in December 2014.
The Phase 2 expansion portion of this Phase 1/2 clinical trial is ongoing, screening patients for EphA3 expression, and is currently focused on patients diagnosed with either myelofibrosis or myelodysplastic syndrome.
KaloBios is evaluating other potential oncology indications for KB004, including additional hematologic malignancies and solid tumors.
Please refer to the KB004 Program Fact Sheet for additional program information.
Disclaimer: The information contained hereto concerns biologics that are under pre-clinical and/or clinical investigation and which have not yet been approved for marketing by the U.S. Food and Drug Administration (FDA). They are currently limited by Federal law to investigational use, and no representations are made as to their safety or effectiveness for the purposes for which they are being investigated.
KB004 Fact Sheet

Humaneered® Antibody Targeting EphA3 for the Treatment of Cancer